According to a new study, a newly developed monitoring device for heart failure patients may detect signs of fluid overload. This device could be used to monitor patients more effectively and help prevent hospital admissions.
The research – which was presented at the European Society of Cardiology’s Heart Failure Congress in Lisbon on May 12th and published in the European Journal of Heart Failure – suggests that costly hospital admissions in heart failure patients could be prevented if fluid accumulation in the heart could be detected earlier to allow for medical intervention aimed at reducing fluid overload.
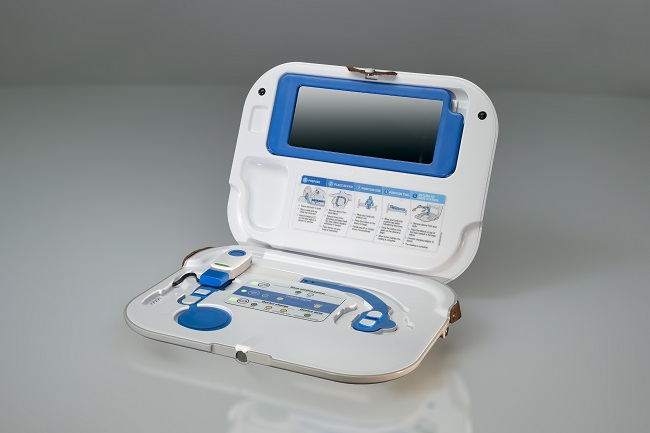
Currently, patients with heart failure are admitted to hospital many times during their disease for treatment with intravenous (IV) diuretics for the relief of congestion; and current methods to detect congestion rely on expensive, invasive monitoring through functions in specially designed pacemakers or by monitoring pressures in the lung using a sensor implanted directly in the lung.
Keen to find a viable alternative to current methods, the research team trialled the use of the non-invasive, Sensinel™ Cardiopulmonary Management System, from Analog Devices Inc (ADI), which uses multiple sensors to measure a number of physiological parameters. They found that the device was able to detect changes in fluid in patients with heart failure who had been admitted to hospital to receive fluid removal, either by decongestion therapy or haemodialysis.
Overall, the results showed that the device performed well, and was also additionally able to detect the changes in fluid and weight in patients as they had fluid removed. The researchers believe that this new wearable sensor device, worn by patients for less than five minutes each day, shows promise as a method of monitoring patients with heart failure and detecting fluid overload, and potentially reducing hospital admissions.
Study lead, Dr James Curtain, Honorary Clinical Research Fellow at the University of Glasgow’s School of Cardiovascular & Metabolic Health, said:
“We found that the Sensinel CPM system was able to detect important changes in the fluid status in our patients. Many of the measurements taken by the device were able to detect changes in our patients as they had fluid removed.”
Senior author on the study, Professor Pardeep Jhund, Professor of Cardiology and Epidemiology at the University of Glasgow, said:
“We are very excited by the results of our study using the Sensinel CPM system in patients with heart failure. This innovative system captures numerous vital patient measurements, aligning closely with the metrics we rely on in clinical practice to identify fluid overload.
“As the device is designed to be used by patients at home, we hope that in the future that we can give the device to patients and detect fluid accumulation early, thereby allowing us to alter their medication and prevent them from needing a costly hospital admission. As the device is only worn for less than five minutes twice a day this could be a real alternative to expensive implanted monitors or monitors that have to be worn all the time.
“The next step will be to conduct a large trial to determine if the device can detect fluid accumulation in patients who use it at home and provide sufficient warning so that we can reduce hospitalisations in those patients who use the device.”
Dr Tony Akl, Senior Director of Biomedical and Clinical at ADI, said:
“The study aims to validate non-invasive measurements that can identify warning signs for patients at risk before hospitalisation is needed.”
Dr Venu Gopinathan, Fellow and Managing Director of Medical Products Group at ADI said:
“The collaboration with University of Glasgow facilitated a critical clinical oversight that allowed us to choose the right physiological parameters to monitor.”
For the study – COrelation of the Non-invasive CardioPulmonary Management wearable device with measures of conGESTion in Heart Failure study (CONGEST-HF) – 66 patients were included, including those who were admitted to the Queen Elizabeth University Hospital (QEUH), Glasgow, and the Scottish National Advanced Heart Failure Unit. The device is applied to the skin of the patient’s chest, and the device communicate via Bluetooth with a mobile app providing patients and health care professionals with information on the health of the patient.
CONGEST-HF was sponsored by NHS Greater Glasgow and Clyde Research and Innovations, and ADI provided funding to the University of Glasgow for the design and conduct of the study.
The paper, ‘Measuring congestion with a non-invasive monitoring device in heart failure and haemodialysis: CONGEST-HF,’ is published in the European Journal of Heart Failure.